Using Chemical Proteomics to Identify the Functional Target SLC25A20 of Ingenol Mebutate for Actinic Keratosis
This study utilizes chemical proteomics to unveil a novel target of macromorol methyl butylate (Ing-Meb), a drug employed in treating solar keratosis. The research team initially devised and synthesized an Ing-Meb photoaffinity probe named Ing-Dayne, which forms covalent bonds with target proteins upon UV light exposure, facilitating the identification of potential functional targets. Through subsequent validation, the authors identified SLC25A20 as a pivotal target of Ing-Meb. SLC25A20 is a mitochondrial membrane-bound carnitine-acylcarnitine translocase involved in fatty acid metabolism. Notably, the inhibitory action of Ing-Meb on SLC25A20 function leads to augmented accumulation of long-chain acylcarnitines, substantiating that Ing-Meb elicits its therapeutic effects by modulating the fatty acid oxidation pathway. Furthermore, the study delves into the significance of structural modifications in natural product investigations. By synthesizing the Ing-Dayne photoaffinity probe through structural modification of Ing-Meb, the researchers successfully unearthed a novel target distinct from conventional understanding. This not only enhances comprehension of Ing-Meb and its mode of action but also underscores the pivotal role of structural modification in chemical proteomics research for unraveling the complexities of natural products. Such insights further propel drug innovation, encompassing optimization of existing drug efficacy, development of novel therapies targeting specific entities, and elucidation of drug side effects mechanisms.
Research Route
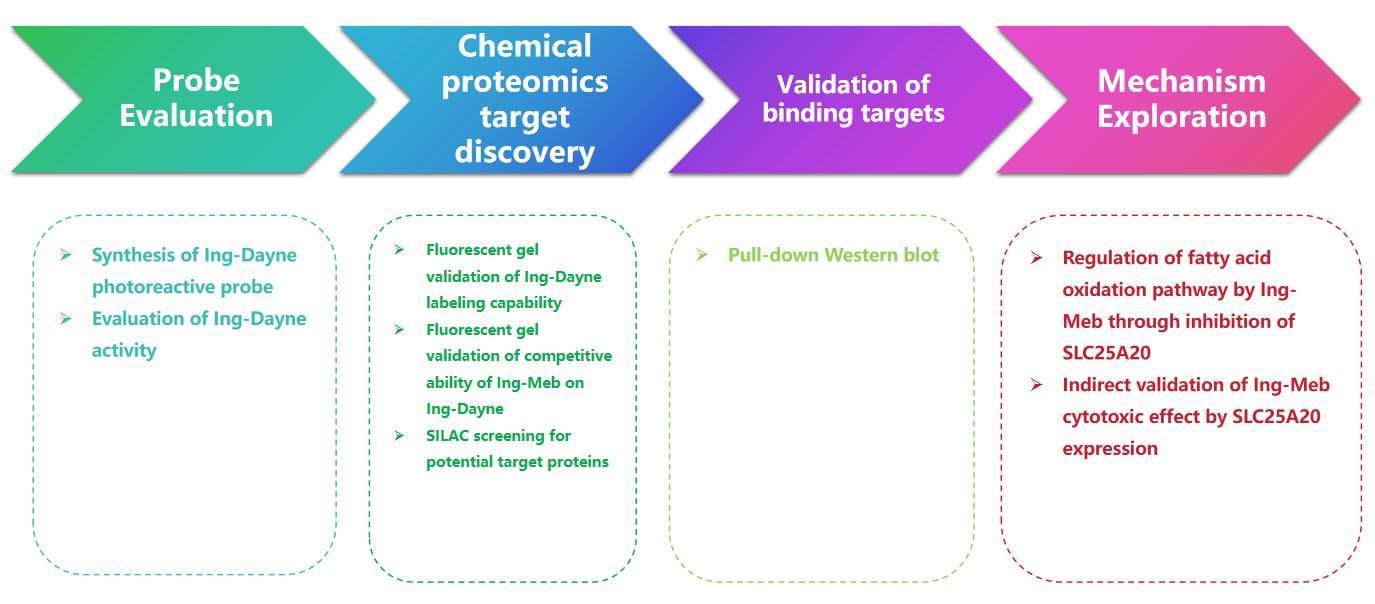
Experimental process
1. Designing the synthetic Ing-Dayne photoaffinity probe unveils the mechanism underlying the binding of the Actinic Keratosis drug Ing-Meb to its target protein.
In this study, the Ing-affinity probe Ing-Dayne, derived from the Actinic Keratosis therapeutic drug Ing-Meb, was employed (refer to Figure 1). Through co-culturing this probe molecule with specific cells and subsequent UV light irradiation, the double acridine structure within the probe formed stable covalent bonds with intracellular proteins. Upon cell lysis, a Click chemical reaction facilitated the attachment of the reporter group azide-tetramethylrhodamine to the target protein (as illustrated in Figure 2). Subsequent SDS-PAGE analysis (depicted in Figure 3) revealed that Ing-Meb exhibited a significant competitive binding effect on the probe, whereas Ingenol, possessing a similar structure, did not demonstrate competitive inhibition.
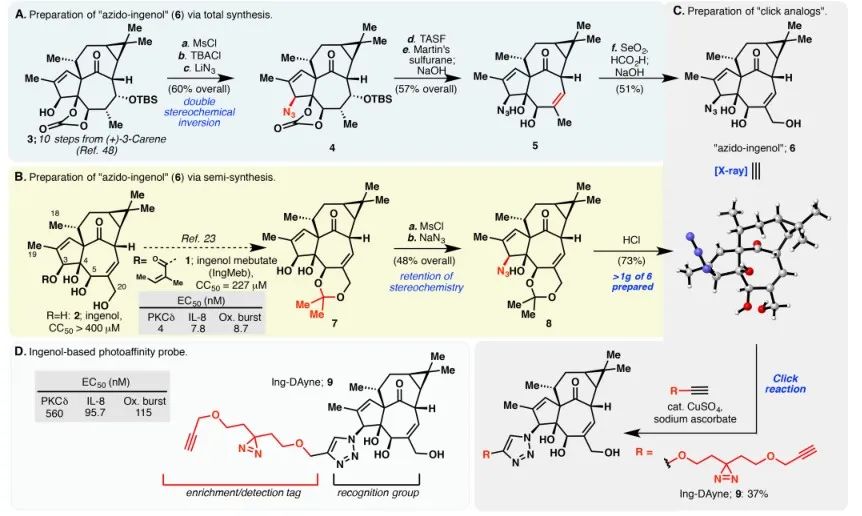
Figure 1: Illustrates the two synthetic routes employed for the synthesis of Ing-Dayne.
2. The novel probe 11b has identified 44 inflammation-related target proteins of BBR within THP-1 cells, and it has revealed EIF2AK2, eEF1A1, PRDX3, and VPS4B as direct targets with specific interactions with BBR.
The authors, through a series of experiments, successfully employed the novel probe 11b to tag and purify potential target proteins within THP-1 cells. Following this, they utilized LC-MS/MS analysis to identify 44 inflammation-associated proteins in the molecular weight range of 20 to 80 kDa, among which six were found to potentially play critical roles in BBR's anti-inflammatory actions. In further investigations, EIF2AK2, eEF1A1, PRDX3, and VPS4B were confirmed as direct targets of BBR, exhibiting competitive inhibition effects under high concentrations of BBR treatment. This finding disclosed the likely existence of specific interactions between these proteins and BBR, thereby elucidating new insights into their engagement with the drug during its anti-inflammatory processes.
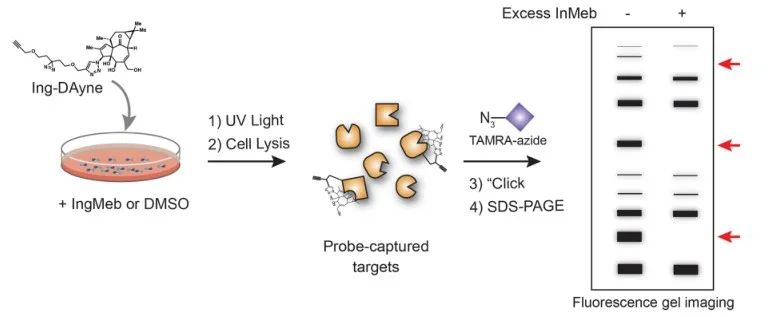
Figure 2: Gel-based validation process of Ing-Dayne target.
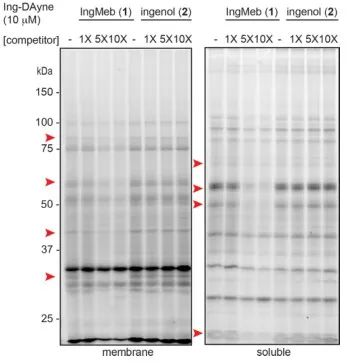
Figure 3: Competitive inhibition of Ing-Meb and ingenol on the probe molecule Ing-Dayne.
3. SILAC screening, combined with Western blot validation, identified SLC25A20 as a primary target of the Ingenol-class drug Ing-Meb.
The authors initially screened 28 potential target proteins using Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) technology (Figure 4). Subsequently, through further screening and Western blot validation experiments (Figure 5), the researchers observed that Ing-Meb exhibited the strongest inhibitory effect on SLC25A20 among these candidate targets, strongly suggesting that SLC25A20 is one of the core functional targets of Ing-Meb.
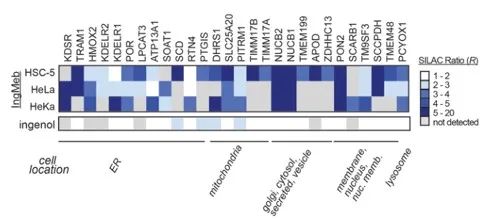
Figure 4: The 28 potential targets obtained by the initial screening.
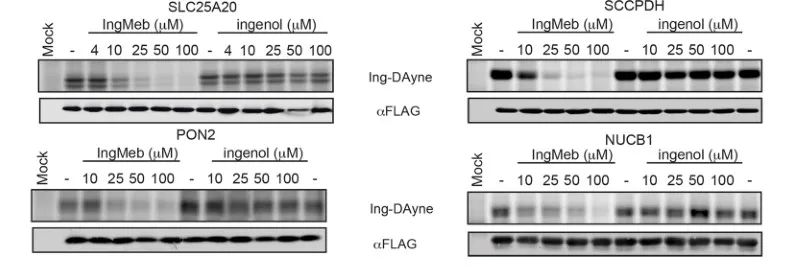
Figure 5: Western blot experiments of four potential target proteins.
4. Ing-Meb regulates the fatty acid oxidation pathway by inhibiting SLC25A20, a mitochondrial membrane protein also known as botulinum alkalilipoyltransferase, thereby revealing its target as an Actinic Keratosis drug.
The authors investigated SLC25A20, also known as botulinum alkali lipoyltransferase or CACT, a multimodal integral membrane protein localized within the mitochondrial membrane. Its primary function involves the transport of long-chain acylcarnitines into the mitochondria, facilitating their exchange with free carnitine. These transported acylcarnitines are subsequently converted into fatty acylCoA by carnitine palmitoyltransferase-2 (CPT-2), thus serving as precursors for fatty acid β-oxidation. Experimental findings revealed a significant increase in cellular long-chain acylcarnitine content with escalating concentrations of the Actinic Keratosis drug Ing-Meb (depicted in Figure 6). This observation strongly supports Ing-Meb's inhibitory effect on SLC25A20, thereby confirming SLC25A20 as one of the principal targets mediating the action of Ing-Meb.
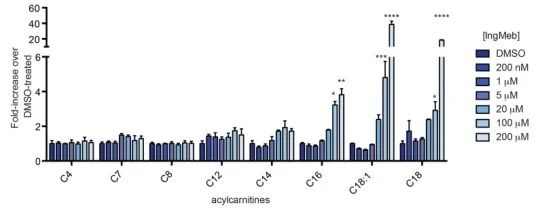
Figure 6: Effect of Ing-Meb for long-chain acylcarnitines
5. Indirect Validation of Ing-Meb Cytotoxic Effect via SLC25A20 Expression: Revealing Its Target Function in Drug Activity Regulation.
The investigators sought to directly link the inhibition of SLC25A20 in HeLa cells with the potential cytotoxic effects of Ing-Meb. The results indicated that while overexpression of SLC25A20 did not alter the half inhibitory concentration of Ing-Meb in these cells, it is noteworthy that increased SLC25A20 expression significantly mitigated the impact of Ing-Meb on long-chain acylcarnitine accumulation (depicted in Figure 7). This outcome further substantiates the hypothesis that SLC25A20 serves as a pivotal functional target for the drug activity of Ing-Meb.
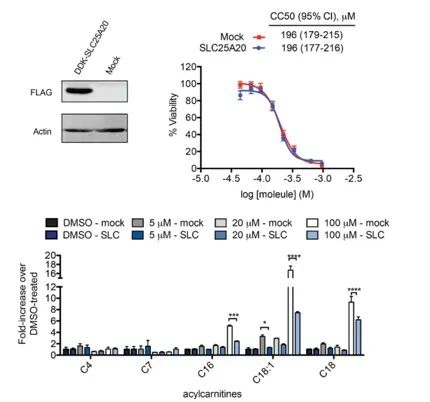
Figure 7: Effect of expression or not of SLC25A20 on CC50 and acylcarnitine.
This study not only reveals the new targets of Ing-Meb, but also provides a new theoretical basis and strategic direction for the target discovery of structurally complex natural products. Reference:
Reference: https://doi.org/10.1021/acscentsci.7b00420.

