[High-Scoring Breakthrough] Advancing Anti-Inflammatory Regulation of SLC15A4 with Novel Inhibitor AJ 2-30: A Chemical Proteomics Approach (IF 14.8)
This article employs chemical proteomics to devise and screen a range of novel inhibitors targeting SLC15A4, a crucial inflammatory transmembrane protein highly expressed in antigen-presenting cells. Initial targeting of potential inhibitors utilized the fully functionalized fragment (FFF) probe library strategy, identifying FFF-21 as a potent inhibitor of IFN-α production among numerous probes. Subsequent structural optimization led to the development of compound AJ 2-30, which not only demonstrated outstanding inhibition but also confirmed SLC15A4 as its primary target post-photocrosslinking modification. Further investigations revealed that AJ 2-30 exerted potent anti-inflammatory effects by reducing SLC15A4 stability, inducing its degradation via the lysosomal pathway, and effectively inhibiting the activation of TLR 7-9 and NOD. This study not only successfully pioneers SLC15A4 inhibitors with significant anti-inflammatory activity but also paves the way for the development of novel anti-inflammatory drugs.

Research Route
Experimental process
1. Fully functionalized fragment (FFF) probe library strategy to screen for SLC15A4 inhibitors.
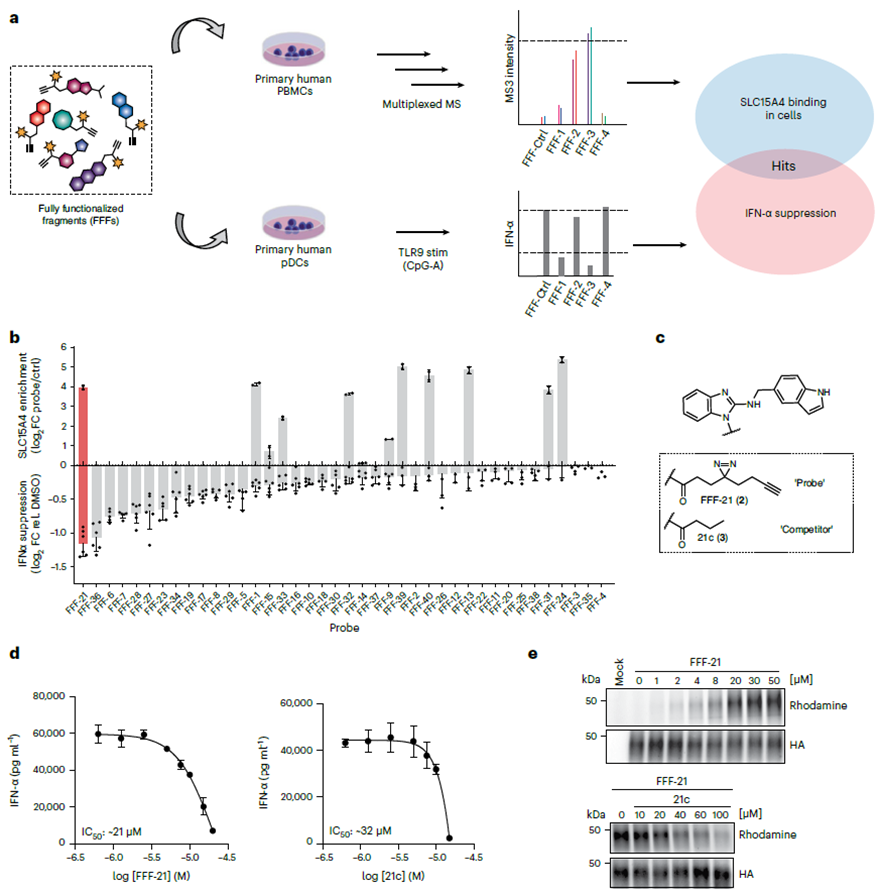
The authors initiated their study by screening SLC15A4 inhibitors using a fully functionalized fragment (FFF) probe library. This FFF comprises two components: one molecular recognition group containing tablet fragments designed to target SLC15A4, and another photo-crosslinked enriched group featuring double acrimidine and alkyne groups to visualize SLC15A4. Employing a chemical proteomics workflow, the authors confirmed the binding of this FFF probe library to SLC15A4 in cells and assessed whether the FFF probe could attenuate IFN-α levels in TLR 9-stimulated cells. Results demonstrated that among all FFF probes binding to SLC15A4, FFF-21 exhibited the highest capacity to inhibit IFN-α levels, with its label-free analogue, FFF-21c, also displaying similar inhibitory effects.
2. Photocross-linking modification of the SLC15A4 inhibitors.
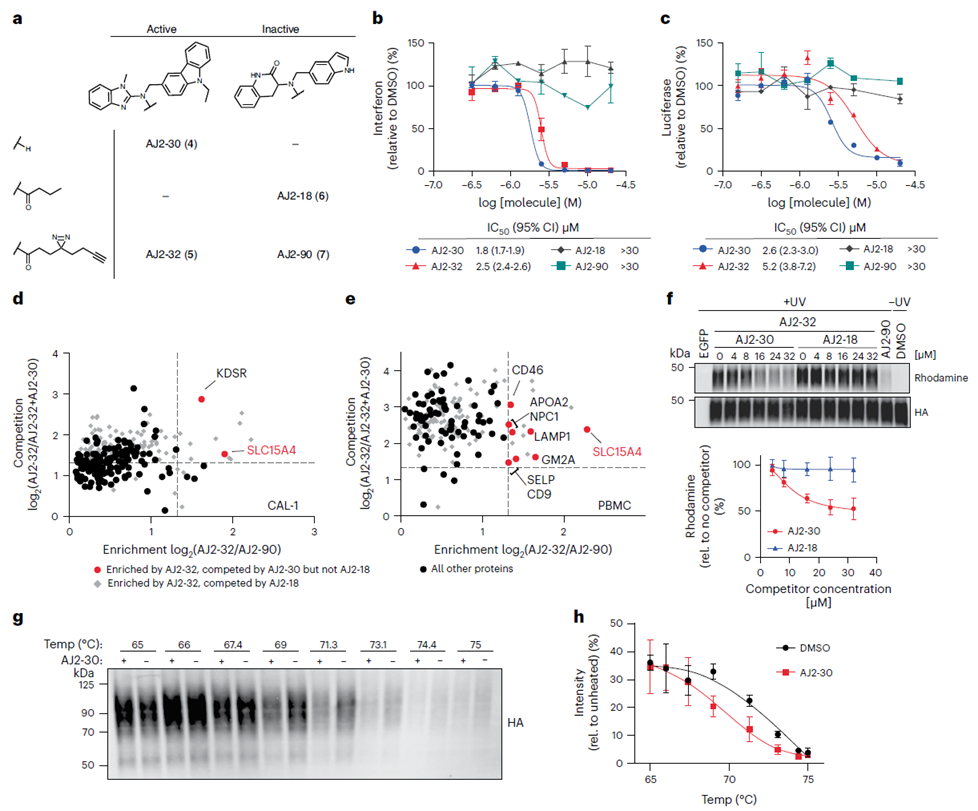
Following the initial selection of compound FFF-21c, the authors proceeded to optimize its structure. Through the synthesis of several analogues of FFF-21c, they identified AJ 2-30 as the most potent inhibitor. Employing photocrosslinking techniques, they confirmed its interaction with the target. Subsequently, utilizing both the direct labeling method and the indirect competition method of ABPP, along with cellular thermal shift analysis (CETSA), the authors definitively established SLC15A4 as the primary target of AJ 2-30.
3. Anti-inflammatory mechanism reveals that AJ 2-30 disrupts SLC15A4 stability.
Following confirmation of AJ 2-30's binding to SLC15A4 and its inhibitory effect, the authors conducted a series of biochemical experiments to elucidate the anti-inflammatory mechanism of AJ 2-30. Results revealed that AJ 2-30 induces lysosome-mediated degradation of SLC15A4 by destabilizing the protein, thereby inhibiting the activation of TLR 7-9 and NOD in immune cells in a SLC15A4-dependent manner. This underscores the potential of AJ 2-30 in treating inflammatory and autoimmune diseases.
In summary, this study adeptly utilizes chemical proteomics techniques to identify AJ 2-30, a potent SLC15A4 inhibitor with remarkable anti-inflammatory activity. This compound demonstrates significant efficacy in animal models by specifically targeting SLC15A4 stability and its role in antigen presentation and inflammatory signaling pathways. This pivotal advancement not only enhances our understanding of anti-inflammatory therapeutic targets but also lays a robust foundation for the future development of novel therapeutic agents for a variety of inflammatory and autoimmune diseases. With further research and clinical validation, these new SLC15A4 inhibitors are poised to offer more precise and effective treatment options for a multitude of patients, thereby making a profound impact on global health.

