Contesto tecnico
La tecnologia proteomica chimica emerge come un faro di cambiamento, indirizzando la scoperta di farmaci lontano dalle proteine purificate e verso il regno delle cellule viventi.
La tecnologia della proteomica chimica funge da solida piattaforma per la scoperta di farmaci, caratterizzata dallo studio delle interazioni tra piccole molecole e proteine all'interno delle cellule viventi. Una delle nostre piattaforme di scoperta di farmaci covalenti è progettata per scoprire leganti covalenti che coinvolgono bersagli proteici attraverso reazioni covalenti con il gruppo tiolico dei residui di cisteina. A differenza delle tradizionali strategie di screening a bersaglio singolo, la piattaforma di proteomica chimica consente l’analisi quantitativa delle interazioni tra frammenti elettrofili e quasi tutte le proteine nelle cellule viventi con la risoluzione dei residui di amminoacidi. Vanta una copertura di oltre 10.000 proteine e~40.000 siti di cisteina (potenziali siti di legame di farmaci) da diverse linee cellulari di mammiferi.

Flusso di lavoro
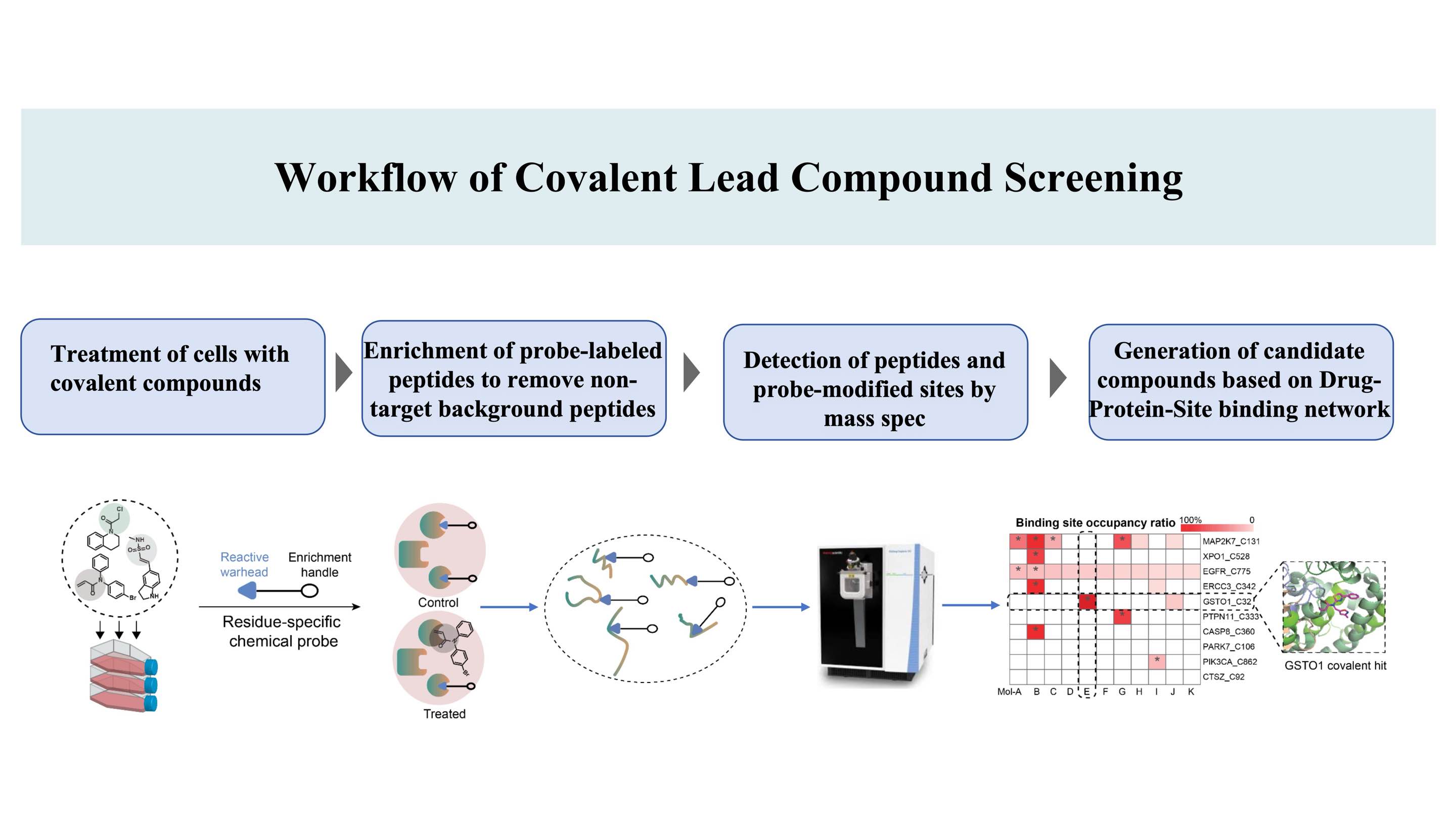
Il flusso di lavoro per la scoperta di composti covalenti mirati ai siti di legame*sulle proteine, basato sul brevetto DIA-ABPP (Data-Independent Acquisition-Activity-Based Protein Profiling) (un sito di legame covalente è un amminoacido che può essere marcato da sonde chimiche, consentendo così la ligabilità)
Vantaggi tecnici
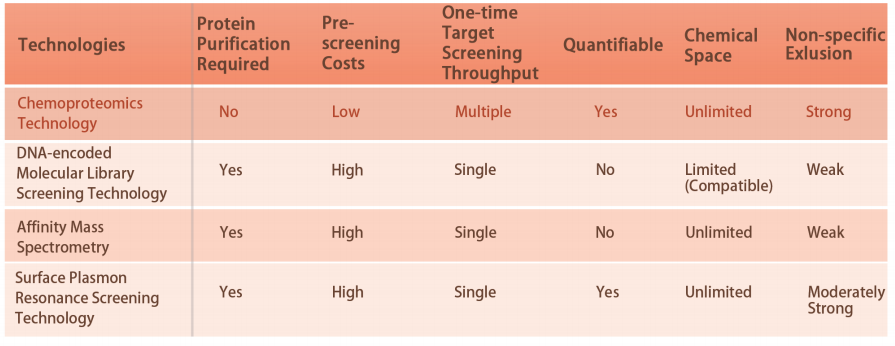
Caratteristica 1
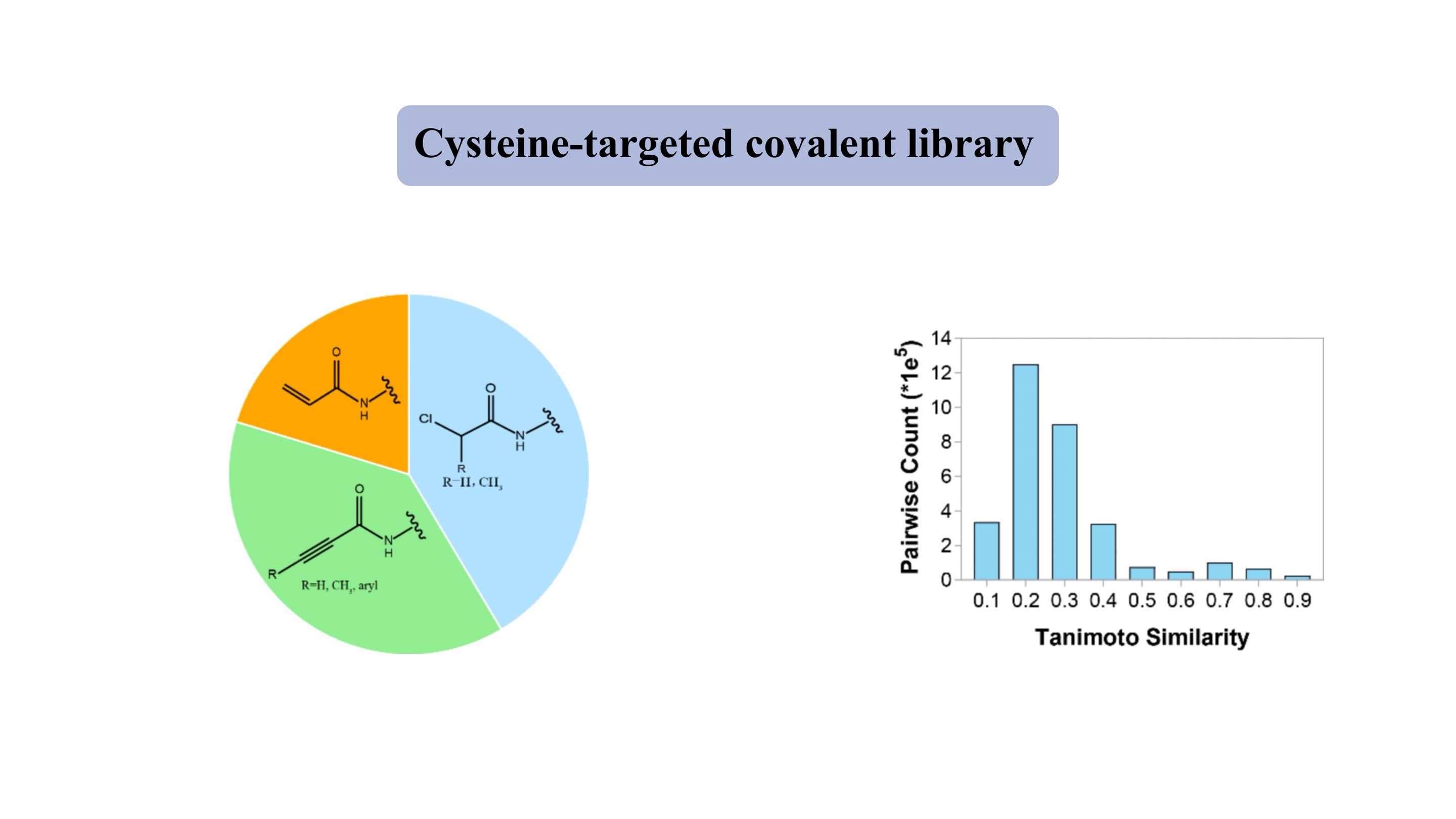
Libreria covalente mirata alla cisteina
La libreria covalente mirata alla cisteina contiene “testate” elettrofile leggere rappresentative, come acrilamidi e cloroacetamidi. La libreria “simile ai farmaci” contiene circa 3000 composti, di cui oltre l’80% presenta un peso molecolare di 300-500 Da. Per la maggior parte dei composti, l'indice di somiglianza Tanimoto è di circa 0,3 per ogni due membri, indicando un elevato grado di diversità.
Caratteristica 2
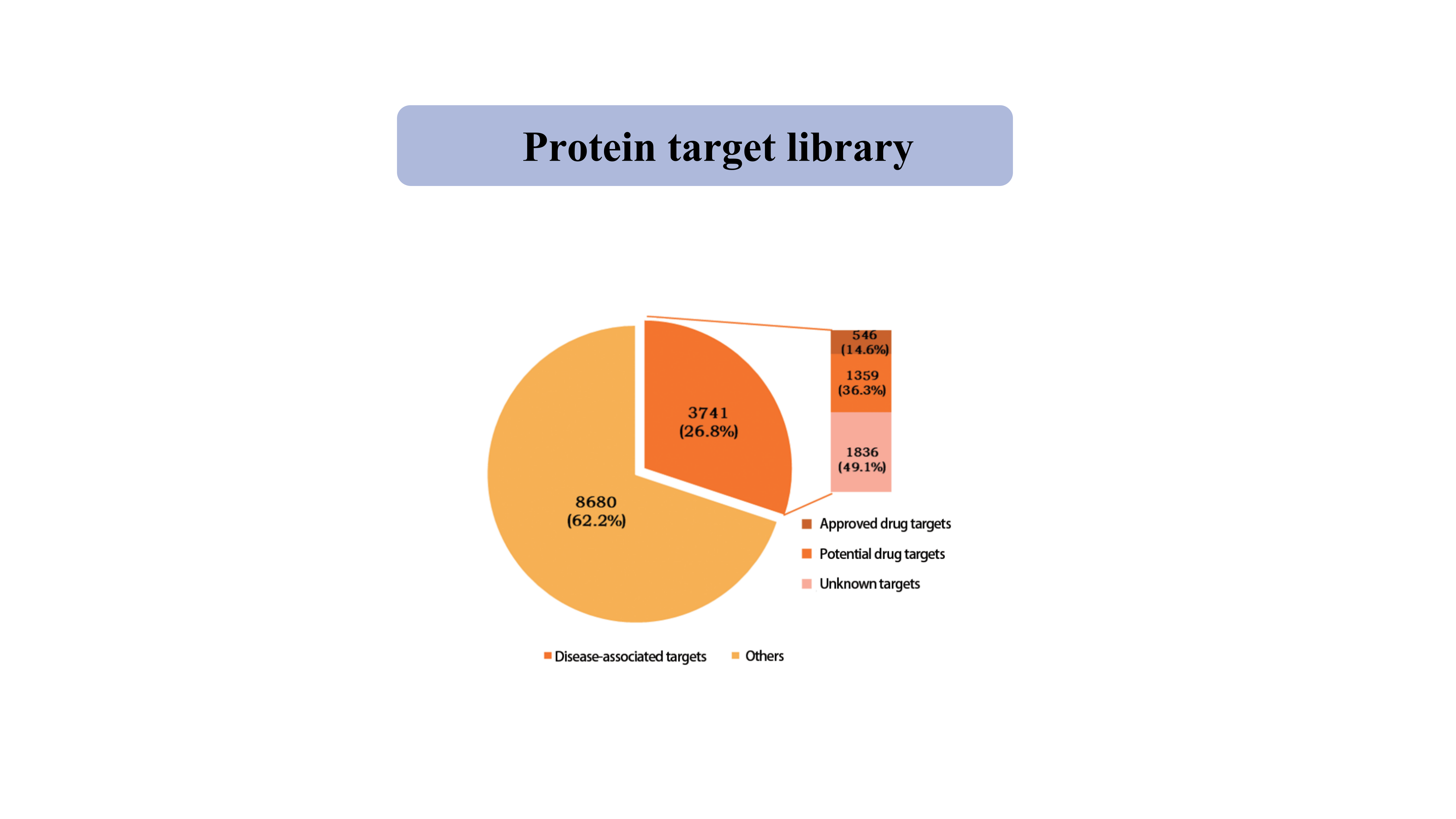
Bersaglio proteicobiblioteca
Attualmente, la libreria di target proteici catturati dalla sonda chimica tiolo-specifica copre 39962 siti di cisteina da 12421 proteine, tra cui chinasi, fosfatasi, ligasi e fattori di trascrizione.
Caso di studio
DCAF1 funge da recettore del substrato per due distinte ligasi E3 (CRL4DCAF1 e EDVP), svolgendo un ruolo fisiologico critico nella degradazione delle proteine. Sono stati sviluppati diversi leganti covalenti e non covalenti mirati al dominio WDR di DCAF1 per supportare applicazioni di degradazione mirate (Targeted Protein Degradation by Electrophilic PROTACs that Stereoselectively and Site-Specifically Engage DCAF1. J. Am. Chem. Soc. 2022, 144, 40, 18688 –18699. PROTAC basati su DCAF1 con attività contro bersagli clinicamente validati superamento delle resistenze degradanti intrinseche e acquisite Comun.
Abbiamo scoperto per la prima volta che Mol-380 interagisce covalentemente con DCAF1_C69, evidenziandolo come un potenziale sito drogabile per applicazioni TPD, separato dal dominio WDR. I nostri risultati evidenziano il valore significativo della piattaforma proteomica chimica automatizzata ChomiX nella scoperta di nuovi ligandi per bersagli non drogabili nelle cellule viventi, inclusi fattori trascrizionali e proteine di membrana, sottolineando il suo potenziale impatto nello sviluppo di farmaci e nell’esplorazione funzionale.
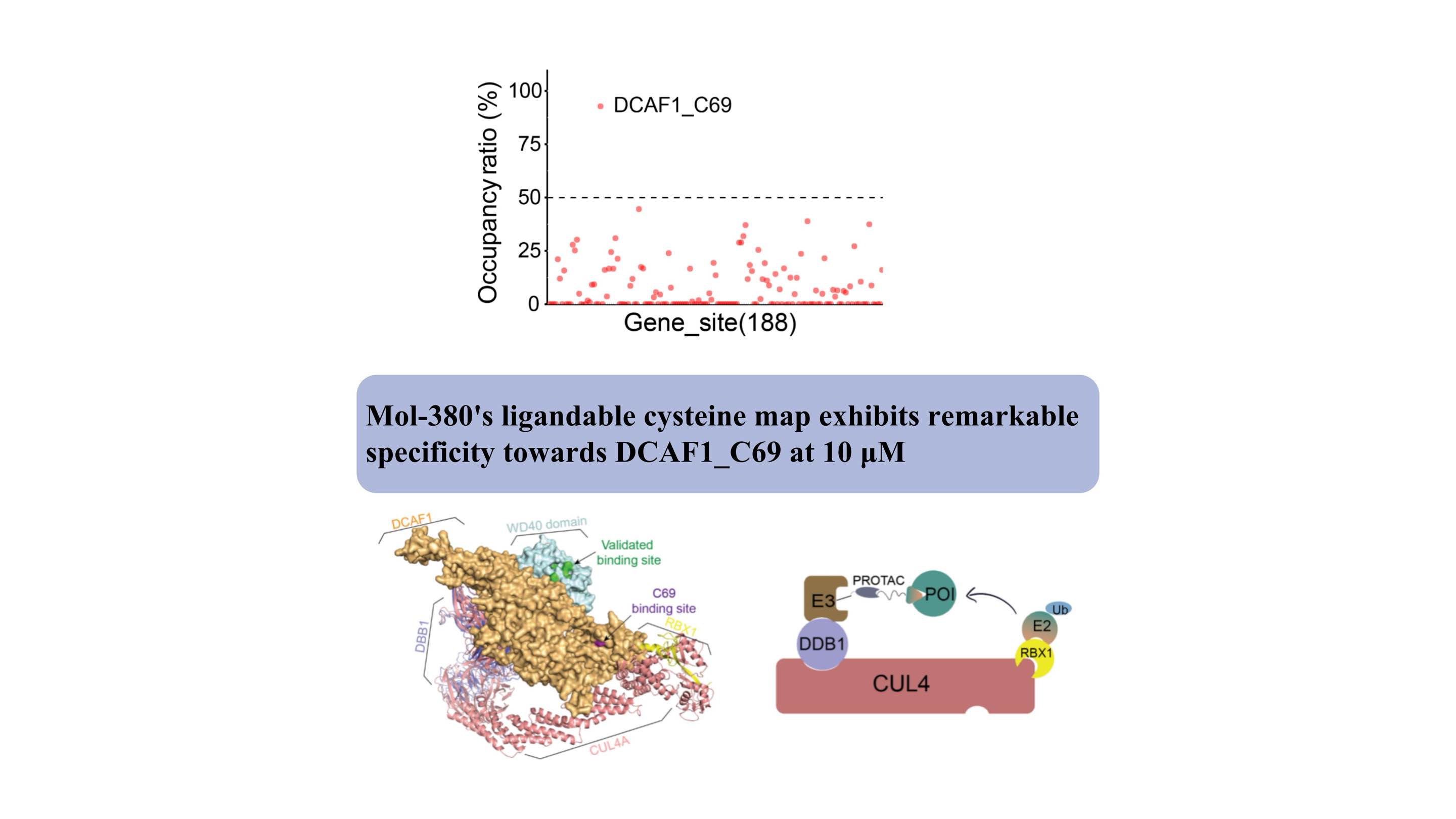
Strutturalmente, il sito di legame di C69 si trova adiacente alla tasca validata sul dominio WD40, come indicato dal modello complesso, offrendo un nuovo sito per lo sviluppo di PROTAC.

