[National Key Project Funding] Unraveling Metal-Binding Proteins: METAL-TPP Breakthroughs and Drug Discovery Innovations
Metal-binding proteins are crucial components that form stable complexes with metal ions, serving various vital functions in living organisms, including cell signaling, catalyzing biochemical reactions, and maintaining metal ion homeostasis. Their dysregulation is associated with diseases like cancer, neurological disorders, and metabolic diseases. Understanding their role in disease pathogenesis is pivotal for diagnosis and treatment. Moreover, metal-binding proteins are significant targets in drug development, as many drugs interact with them to exert therapeutic effects. In this study, researchers introduced METAL-TPP, a state-of-the-art chemical proteomics method. Combining thermostability quantitative proteomic analysis (TPP) with metal-binding protein identification, METAL-TPP enables efficient and accurate detection of metal-binding proteins. Notably, it regulates protein thermostability using a range of metal chelators, facilitating precise identification in pure proteins and cell lysates. This innovative approach offers new insights into metal-binding protein function and mechanisms, aiding biological research, disease understanding, and drug development. Keloxi Biology provides advanced ABPPs, TPP, and other technical services in pharmaceutical R&D, supporting researchers in exploring drug mechanisms and advancing new drug development processes.

Experimental process
1. Seventeen potential metal-binding proteins were identified using METAL-TPP.
Initially, researchers assessed the efficacy of broad-spectrum metal chelation using EDTA on pure proteins and cell lysates. They observed that METAL-TPP effectively detected reductions in thermal stability of metal-binding proteins. Subsequently, a systematic analysis was conducted on human-derived proteins using METAL-TPP, identifying 125 proteins exhibiting decreased thermostability. Among these, 102 were previously known metal-binding proteins. Additionally, 17 potential metal-binding proteins without prior functional annotation were uncovered, offering novel insights into the role of metal-binding proteins.
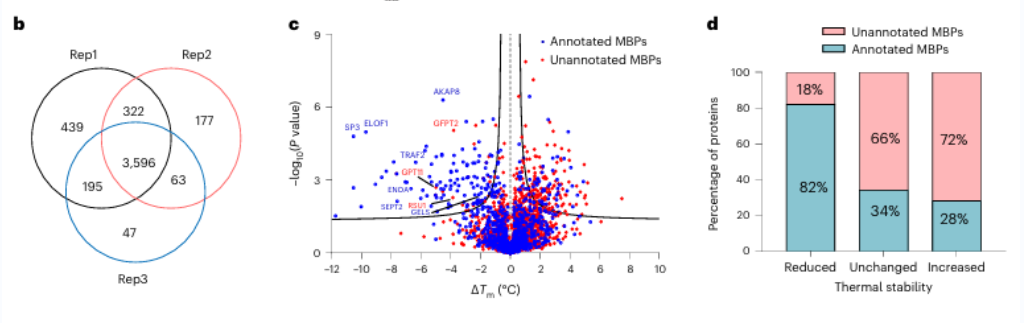
2. The effect of zinc ions on the potential metal-binding protein GFPT2.
Among these 17 potential metal-binding proteins, researchers chose to conduct in-depth biochemical validation on the protein GFPT2. GFPT1/2 serves as the first rate-limiting enzyme in the hexose biosynthesis pathway, aiding in the generation of a substance called UDP-GlcNAc. Researchers confirmed at the cellular level that zinc ions interact with GFPT2, inhibiting its activity. Additionally, they observed that the presence of zinc ions leads to a significant reduction in UDP-GlcNAc levels, indicating zinc ions regulate the hexose biosynthesis pathway by inhibiting the activity of GFPT2. Interestingly, zinc ions exhibit different selectivity in inhibiting the activities of GFPT2 and GFPT1, suggesting the presence of a novel regulatory mechanism.
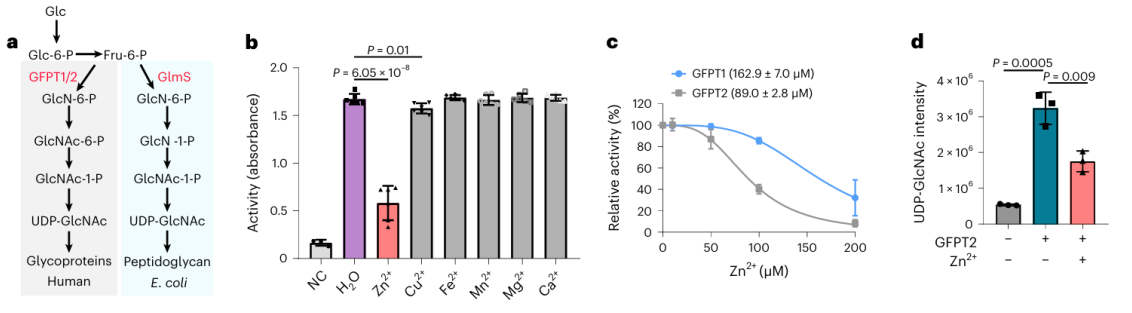
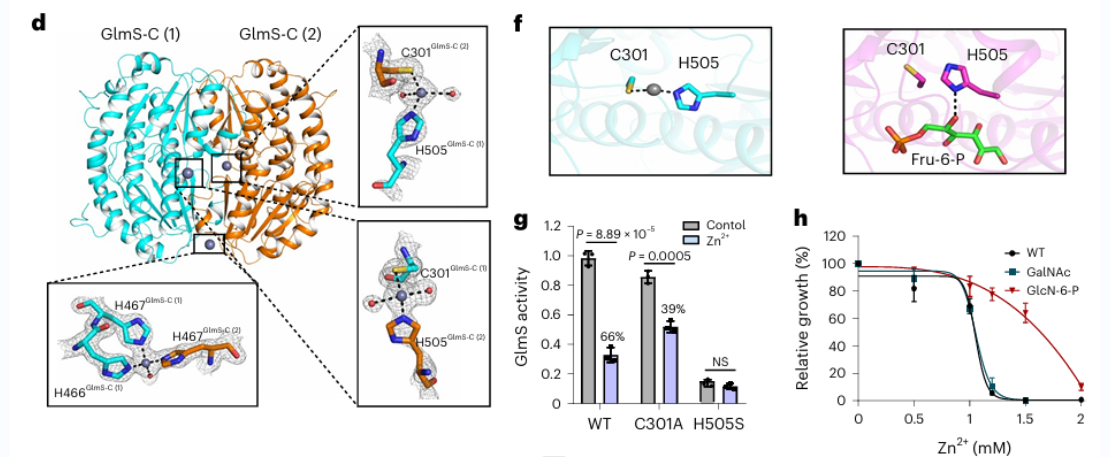
3. Deciphering the molecular mechanism of zinc ions on the activity of GFPT2 and GLMS enzyme.
The researchers isolated the homologue of the GFPT2 protein from E. coli and proceeded with a series of biochemical experiments and crystal structure analyses. The findings revealed that the GLMS protein has the capability to bind zinc ions, and notably, this binding takes place near the substrate-binding region. This suggests a potential scenario where zinc ions could compete for binding at the active site of GLMS and GFPT2, or hinder the activity of both enzymes by forming coordination bonds with their active sites.
4.The metal chelator TPEN can specifically recognize zinc ion-binding proteins.
Finally, researchers extended the ability of METAL-TPP to identify metal-binding proteins in human proteins using the metal chelator TPEN. Experimental results revealed that among the 150 proteins with reduced thermal stability, 110 (73%) were known metal-binding proteins, indicating that TPEN, like EDTA, can specifically recognize metal-binding proteins. Among these, 95 (86%) of the known metal-binding proteins were zinc ion-binding proteins, while only 41% of the proteins with reduced thermal stability caused by EDTA were zinc ion-binding proteins, suggesting TPEN's preference for identifying zinc ion-binding proteins.
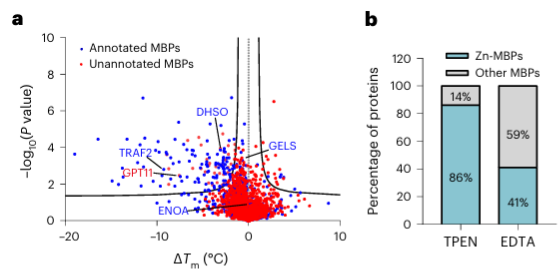
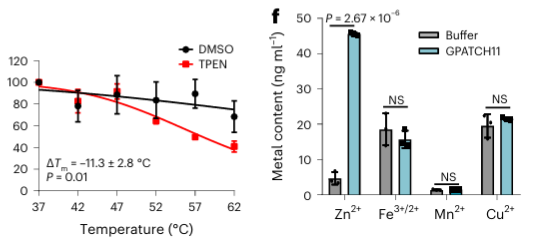
Among the 40 potential metal-binding proteins identified, the authors selected one target protein, GPATCH11, for preliminary biochemical validation and found that this protein is a zinc ion-binding protein.
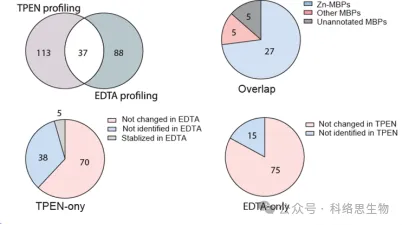
5. Comparison of the recognition ability of two metal chelators in METAL-TPP.
The researchers also compared the ability of two metal chelators, TPEN and EDTA, to identify proteins with reduced thermal stability in METAL-TPP proteomics data. They found that among the 37 proteins identified by both chelators, 27 were known to bind zinc, 5 were known to bind other metals, and 5 were previously unannotated as metal-binding proteins. For proteins whose thermal stability decreased under one chelator and remained unchanged or increased under the other, the researchers suggested two possible reasons for the difference in the range of identification by METAL-TPP. First, each chelator may act as a binding ligand in some proteins, making them more stable and counteracting the destabilizing effect caused by metal binding. Secondly, due to the different molecular structures, the two chelators also have greatly different solubility in water. Thus, future METAL-TPP studies can be performed using other chelators with unique molecular structures to more comprehensively explore the scope of the metalloproteome.
Overall, this study has introduced a novel method, METAL-TPP, which serves as a potent tool for conducting metal-binding proteomics investigations. Through this approach, researchers systematically identified metal-binding proteins and elucidated their roles in biological functions and pathogenesis. This endeavor not only establishes important databases but also provides valuable insights for understanding the biochemical functions and drug development potential of metal-binding proteins, thereby fostering further research in related fields.

