Products
New Therapeutic Prospects for Traditional Medicine: ABPP Technology Unveils Berberine's Novel Anti-Inflammatory Target EIF2AK2
Traditional medicine is being revitalized with new pharmacological applications as advanced techniques such as Activity-Based Protein Profiling (ABPP) shed light on previously unknown therapeutic mechanisms. In this context, ABPP technology has recently revealed a novel anti-inflammatory target of berberine, a compound extracted from traditional Chinese medicinal plants and commonly known as Coptisine or Huanglian in China. The discovery highlights EIF2AK2 as a key player in berberine's anti-inflammatory effects, opening up fresh avenues for its use in the treatment of inflammation-related diseases. This breakthrough underscores the potential for repurposing and optimizing traditional medicines through modern scientific methods.
Berberine, a traditional alkaloid with wide-ranging pharmacological effects including anti-inflammation, hypoglycemia, and cardiovascular protection, has drawn considerable attention. However, its precise molecular mechanisms, particularly in inflammation suppression, remain unclear.
This study fills this knowledge gap using ABPP technology to identify EIF2AK2 as a critical target engaged by berberine for its anti-inflammatory action. The findings deepen our understanding of berberine's mechanism and provide a scientific basis for repositioning berberine and developing new EIF2AK2-targeted anti-inflammatory drugs.
The team employed advanced chemoproteomic methods to systematically investigate berberine's interactions with intracellular proteins, confirming its specific binding to EIF2AK2 and modulation of its enzymatic activity. This influences inflammatory response pathways, effectively inhibiting inflammation progression. This significant breakthrough offers insights into berberine's anti-inflammatory mechanism and supports the development of novel therapies targeting EIF2AK2.
ChomiX provides cutting-edge services like ABPP and CETSA to aid researchers in exploring drug mechanisms and expediting new drug development efforts.
Research Route
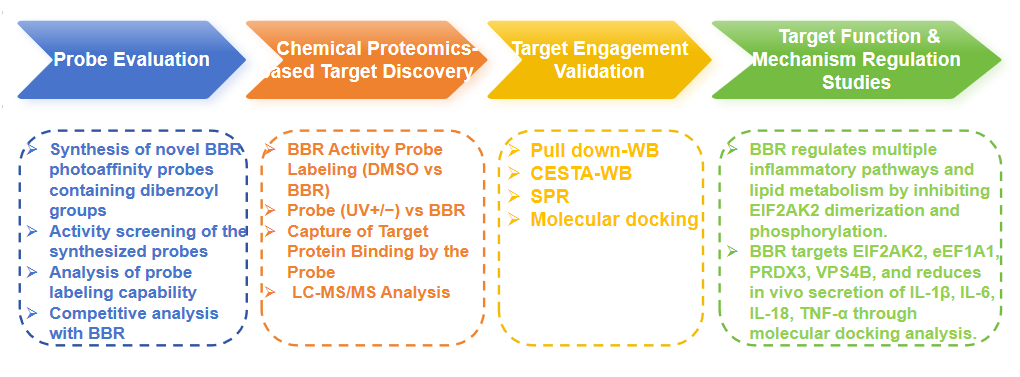
Experimental process
1. Probe 11b was employed as a functional tool in proteomics research.
The authors synthesized and screened novel BBR photoaffinity probes containing dibenzoyl groups within LPS + Nigericin-activated THP-1 macrophages. Among these, compounds 7a and 11b displayed time- and dose-dependent inhibitory effects on IL-1β expression, demonstrating enhanced efficacy compared to the parent compound BBR. Through Activity-Based Protein Profiling (ABPP) analysis and fluorescence scanning, it was substantiated that both 7a and 11b effectively bind to their target proteins and exhibit competitive inhibition, thus indicating a mechanism of action akin to that of BBR. Based on the observation that compound 11b exhibited marked changes in fluorescence intensity within the concentration range of 2.5% to 25%, particularly at a concentration of 20 millimolar where the fluorescence signal variation was most prominent, it was chosen as the functional probe for proteomics studies. This selection was grounded in its superior responsiveness, which renders it suitable for elucidating protein interactions in a proteomic setting.
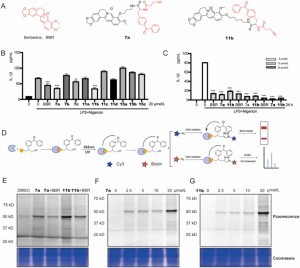
Figure 1: Screening and evaluation of BBR probes.
2. The novel probe 11b has identified 44 inflammation-related target proteins of BBR within THP-1 cells, and it has revealed EIF2AK2, eEF1A1, PRDX3, and VPS4B as direct targets with specific interactions with BBR.
The authors, through a series of experiments, successfully employed the novel probe 11b to tag and purify potential target proteins within THP-1 cells. Following this, they utilized LC-MS/MS analysis to identify 44 inflammation-associated proteins in the molecular weight range of 20 to 80 kDa, among which six were found to potentially play critical roles in BBR's anti-inflammatory actions. In further investigations, EIF2AK2, eEF1A1, PRDX3, and VPS4B were confirmed as direct targets of BBR, exhibiting competitive inhibition effects under high concentrations of BBR treatment. This finding disclosed the likely existence of specific interactions between these proteins and BBR, thereby elucidating new insights into their engagement with the drug during its anti-inflammatory processes.
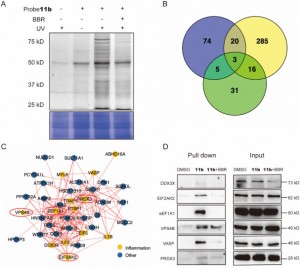
Figure 2: Target proteins capture and functional analysis.
3. Structural biology research elucidates how BBR modulates EIF2AK2 dimerization to exert its anti-inflammatory effects through critical ion interactions and cation-pi binding.
The authors employed CETSA, SPR, and molecular docking techniques to validate the interactions between BBR and four proteins—EIF2AK2, eEF1A1, PRDX3, and VPS4B—in HEK-293 cells. The results showed that BBR increased the thermal stability of these four proteins, with the strongest affinity observed for EIF2AK2. Further investigation revealed that the binding of BBR to EIF2AK2 mainly relies on ion pairs involving D316 and E367 in cavity II, as well as cation-pi interactions with K291. This binding site is involved in EIF2AK2 dimerization, suggesting that BBR might exert its anti-inflammatory effects by modulating EIF2AK2 dimerization.
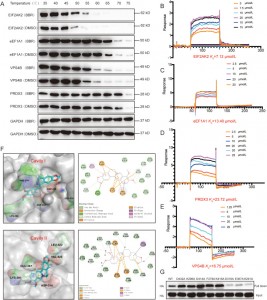
Figure 3: Affinity studies between BBR and its potential targets.
4. BBR's role in inflammation and lipid metabolism pathways is revealed as it inhibits EIF2AK2 dimerization and phosphorylation, thereby demonstrating a pivotal function in regulating the NLRP3 inflammasome, NF-kB p65/JNK/SIRT1 signaling.
Immunoprecipitation experiments confirmed that BBR inhibits EIF2AK2 dimerization, affecting both EIF2AK2 autophosphorylation and phosphorylation of its substrate eIF2a, thus revealing that BBR regulates NLRP3 inflammasome, NF-kB p65, JNK signaling pathways, and SIRT1 expression, playing a crucial role in cellular inflammatory responses, brain anti-inflammatory mechanisms (such as in Alzheimer's disease), and fatty acid-induced endoplasmic reticulum stress. Additionally, silencing or overexpressing EIF2AK2 significantly altered BBR's regulatory effects on p-JNK and SIRT1, further substantiating that BBR acts through binding to EIF2AK2 to regulate inflammation-related lipid metabolic disorders.
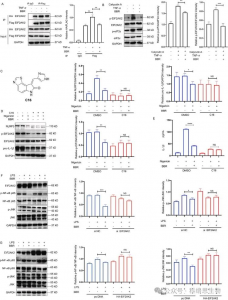
Figure 2 Target proteins capture and functional analysis.
5. By targeting EIF2AK2, eEF1A1, PRDX3, and VPS4B, BBR adjusts multiple inflammatory pathways, with EIF2AK2 playing a dominant regulatory role among these targets.
To explore this interaction further, the authors established overexpression and knockdown models of the four proteins, demonstrating that BBR selectively modulates JNK, NF-kB, MAPK, and AKT inflammatory pathways, with EIF2AK2 playing a dominant role, which was validated in in vivo experiments.
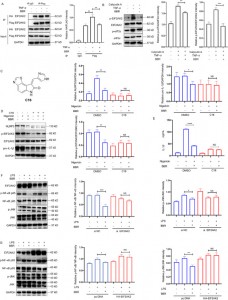
Figure 5: Functional studies of the BBR target proteins
6. Through specific targeting of EIF2AK2, BBR downregulates the in vivo secretion of IL-1β, IL-6, IL-18, and TNF-α; knockdown of the EIF2AK2 gene diminishes its anti-inflammatory efficacy and liver protective actions.
They then investigated whether BBR affects IL-1β, IL-6, IL-18, and TNF-α release by targeting EIF2AK2 in vivo. To do so, they created an EIF2AK2 gene knockout mouse model using intravenous injection of adeno-associated virus (AAV) carrying shEIF2AK2. Wild-type and EIF2AK2 knockout mice were administered BBR (3 mg/kg) intraperitoneally followed by LPS injection. While BBR significantly reduced the levels of IL-1β, IL-6, IL-18, and TNF-α in the control group, this effect was attenuated in the EIF2AK2 knockout group. Histological examination via H&E staining of liver tissue indicated that the ameliorative effect of BBR on liver inflammation infiltration was weakened in EIF2AK2 knockout mice. These findings suggest that BBR potentially downregulates the secretion of IL-1β, IL-6, IL-18, and TNF-α through targeting EIF2AK2 and displays good safety.
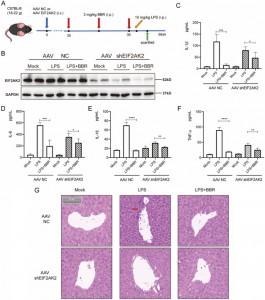
Figure 6: EIF2AK2 functional verifications in vivo.
This study fully demonstrates the powerful advantages of ABPP technology in elucidating the complex mechanisms of bioactive molecules like berberine, propelling the advancement of modern research into traditional drugs. By uncovering new targets and mechanisms of action for the old drug berberine, it not only enriches our understanding of the biological functions of traditional medicines but also opens up fresh perspectives and possibilities for the treatment of inflammation-related diseases. This outcome foreshadows that, with the support of modern scientific techniques such as ABPP, more traditional drugs will be repurposed through the identification of their specific targets and mechanisms, making significant contributions to human health endeavors.
Reference: https://doi.org/10.1016/j.apsb.2022.12.009.
