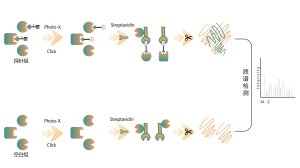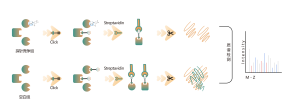Products
Serine/Threonine Post-Translational Modifications
Serine and Threonine Post-Translational Modifications (PTMs) refer to the chemical modifications that proteins undergo after synthesis, with phosphorylation and glycosylation being the most common forms of modification. The PTMs on serine and threonine residues mainly include phosphorylation and O-glycosylation. After modification, these amino acid residues can significantly alter the protein's activity, stability, and interaction capabilities, thereby playing crucial roles in cell signaling transduction, metabolic regulation, and other key biological processes. In-depth studies of these modifications help reveal the molecular mechanisms behind life activities and may provide new targets for disease treatment. Therefore, serine and threonine PTMs hold indispensable importance in both fundamental biological research and clinical applications.
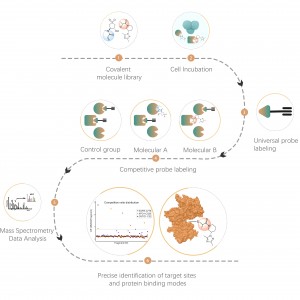
Chomix possesses advanced mass spectrometry technology capable of directly and precisely analyzing various types of protein post-translational modifications and their specific sites. By cleverly combining separation enrichment techniques and isotope labeling, large-scale, high-throughput qualitative and quantitative analyses of various modifications can be achieved, providing robust technical support for in-depth research into protein post-translational modifications.
Technical Platform (O-glycosylation)
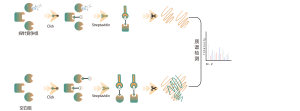
O-linked glycosylation is a biochemical process that transfers sugar chains to the oxygen atoms of serine and threonine residues in peptide chains. Using UDP-GalNAz and Y289L GalT1 reagents, these agents can label sugar molecules on glycosylation sites with an -N3 group. Subsequent steps involving click chemistry, enzymatic digestion, enrichment, and high-resolution mass spectrometry allow for the precise identification of O-glycosylation sites, thereby revealing the crucial role of this post-translational modification in organisms.

Our Advantages
1. Professional Expertise: With extensive experience and publications in leading journals, we offer tailored services for optimal results.
2. Rigorous Quality Management: Our mature quality systems adhere to ISO9001 standards, ensuring reliable reports.
3. Comprehensive Service: From probe design to bioinformatics analysis, we provide all-in-one consultation to delivery, with timely progress updates.
4. Advanced Equipment: Equipped with cutting-edge mass spectrometers like Thermo Fisher Orbitrap Exploris 480 and Bruker timsTOF, we support breakthrough research.
Our Service
| Project | Serine/Threonine Post-Translational Modifications Based on High-Resolution Mass Spectrometry |
| Sample | Lysate, Live Cells, Tissue Samples |
| Hardware Platform | VanquishNeo UPLC coupled with Orbitrap Exploris 480 mass spectrometer (Thermo Fisher Scientific); EASY-nLC1200 UPLC coupled with Q Exactive HF-X mass spectrometer (Thermo Fisher Scientific) |
| Project Duration | 4-8 weeks |
| Deliverables | Project Report (Including gel images, site information, etc.) |
| Price | Click to consult |
Case Study
Project Objective: The aim of this project is to conduct in-depth chemical proteomic analysis of O-GlcNAcylation post-translational modification using mass spectrometry technology.
Solution: We employ a combined approach of metabolic labeling and mass spectrometry to perform precise and comprehensive qualitative analysis of O-glycosylation sites. In the experimental process, we first determine the quantity of Y289L GalT1 enzyme using fluorescence gel technology, followed by mass spectrometric analysis under rigorously defined experimental conditions. Through two rounds of repeated experiments, a total of 36 reliable glycosylation sites were identified. An example of a typical MS2 spectrum is presented.