Unveiling the Mystery of the Small Molecule WA: Shedding Light on PHGDH Enzyme Regulation and Paving the Way for Novel Anti-Cancer Therapies
Serine, an indispensable amino acid, fulfills various crucial biological functions within living organisms. It not only serves as a fundamental component in protein synthesis but also participates in the regulation of numerous metabolic pathways, including nucleotide synthesis, methionine metabolism, and antioxidant functions. Among these pathways, phosphopyruvate dehydrogenase (PHGDH) holds pivotal significance as it catalyzes the initial step of the serine synthesis pathway, converting 3-phosphoglycerate to 3-phosphohydroxypyruvate. Given its central role in serine metabolism, any aberration in PHGDH function is intricately linked to the onset and progression of numerous diseases, particularly cancer.
This article introduces an innovative approach that utilizes chemical proteomics and phenotypic analysis techniques to identify a compound capable of covalently inhibiting PHGDH. While most PHGDH inhibitors are typically competitive in nature, the authors present Withangulatin A (WA), a natural small molecule, as a novel covalent inhibitor of PHGDH. WA emerges as a promising lead compound for the development of PHGDH inhibitors. Moreover, WA serves as a valuable probe for investigating the functionality of PHGDH and the serine synthesis pathway (SSP). Leveraging this inhibitor, researchers gain deeper insights into the regulatory mechanisms governing serine metabolism, thereby opening avenues for exploring potential treatment modalities for related diseases, notably cancer.
This discovery not only offers promising directions for the development of novel drug treatment strategies but also furnishes crucial insights into the role of serine metabolism in disease progression.
Research Route
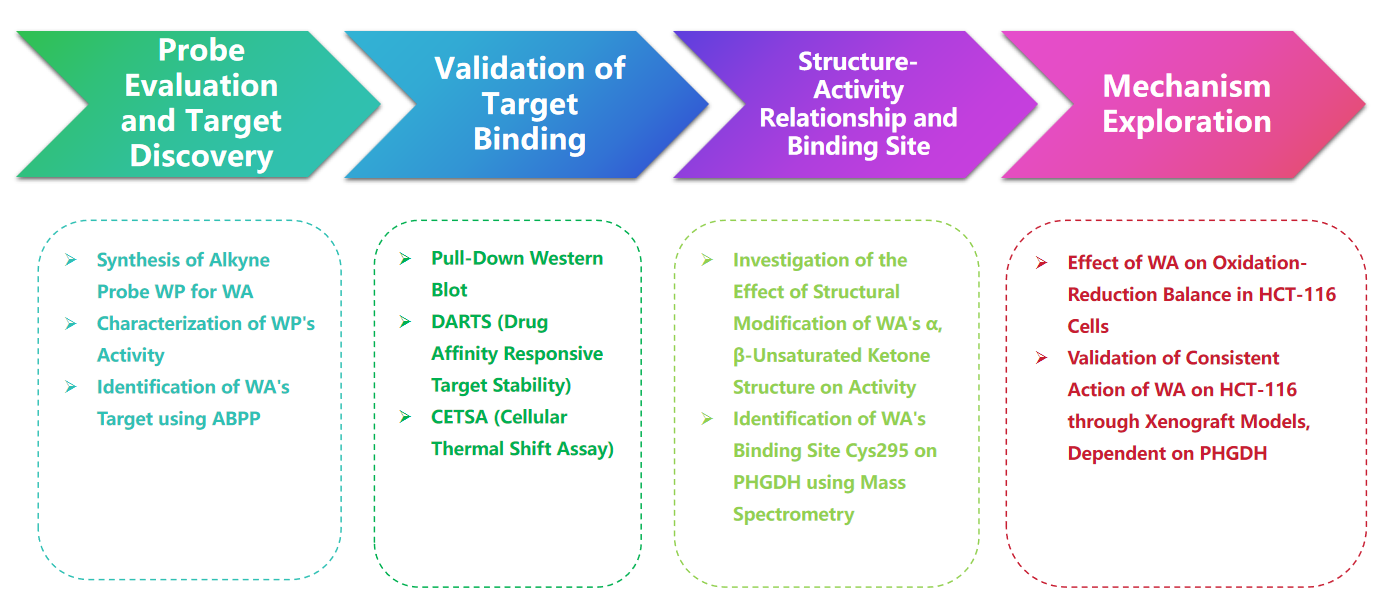
Experimental process
1. Comparison of WP and WA in the cytotoxicity experiments.
In the study, the author devised and synthesized a compound probe termed WP. Employing human colon cancer cells (HCT-116) and normal colon cells (NCM460), the authors observed that WP exhibited comparable cytotoxicity to WA. This suggests that the inclusion of alkyne markers did not significantly alter its cytotoxic effects. Furthermore, the authors noted that WA displayed lower cytotoxicity in normal colon cells but higher cytotoxicity in colon cancer cells, underscoring its enhanced selectivity towards colon cancer cells.
Subsequently, the authors employed an Activity-Based Protein Profiling (ABPP) strategy, utilizing the WP probe in HCT-116 cells. This approach led to the identification of PHGDH as a direct target protein of WA. Experimental validation was carried out using WB-pull down assays, confirming the findings.
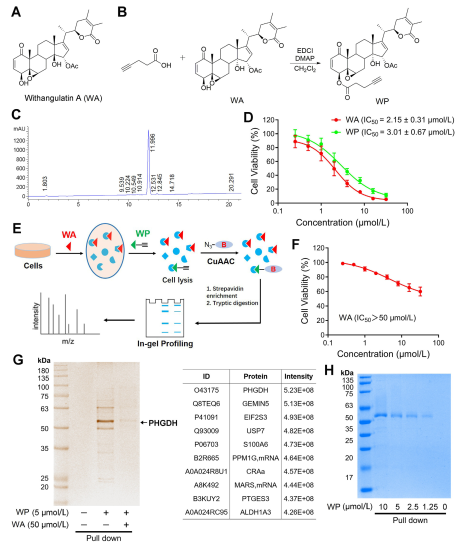
Figure 1: Chemical Proteomics Approach to Identify Targets of Withangulatin A .
2. Confirmation of Direct Interaction between WA and PHGDH.
Utilizing Activity-Based Protein Profiling (ABPP) technology, the authors identified PHGDH as a direct target of compound WA in HCT-116 cells. To validate the interaction between WA and PHGDH, the authors conducted Drug Affinity Responsive Target Stability (DARTS) and Cellular Thermal Shift Assay (CETSA) experiments. The results demonstrated that WA enhanced the thermal stability of PHGDH and significantly inhibited its activity. Furthermore, Biolayer Interferometry (BLI) experiments provided further confirmation of the direct interaction between WA and PHGDH.
The authors also investigated the irreversible nature of WA binding to PHGDH. Their experiments revealed that PHGDH prevented WP binding; however, preincubation with solutions containing N-acetyl cysteine (NAC) or glutathione (GSH) could reverse the binding of WP to PHGDH. These findings suggest an irreversible covalent binding between WA and cysteine residues in the PHGDH protein.
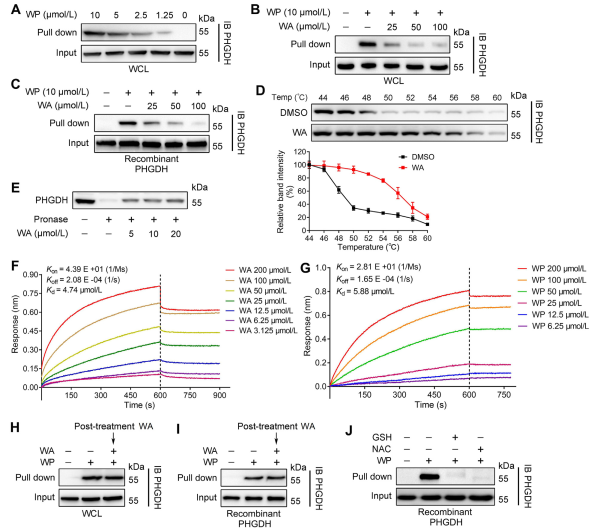
Figure 2: WA Directly Binds to PHGDH.
3. WA covalently binds to PHGDH via the α - β -unsaturated ketone moiety.
To further elucidate the mechanism by which WA interacts with PHGDH, the investigators conducted experiments to confirm the covalent binding of WA to PHGDH through its α-β-unsaturated ketone structure. Initially, WA's α,β-unsaturated ketone fragments were reduced to produce WA-1 (refer to Part Figure 3A). Subsequent analyses using Cell Counting Kit-8 (CCK-8) assays and Pull-down protein interaction assays (refer to Figure 3B) demonstrated that WA's cytotoxic effect was indeed dependent on its α and β-unsaturated ketone structure. Conversely, WA-1 failed to effectively prevent the binding of PHGDH to WP (refer to Figure 3C and D), thereby further confirming the covalent binding of the β-unsaturated ketone to PHGDH (refer to Figure 3E).
Given the significant role of PHGDH inhibition or absence in blocking serine synthesis pathway (SSP) processes in tumor cells, the authors proceeded to investigate the effect of WA on SSP activity in HCT-116 cells using U-13C-glucose stable isotope labeling (refer to Figure 3J). Experimental results revealed that WA effectively inhibited SSP activity in HCT-116 cells.
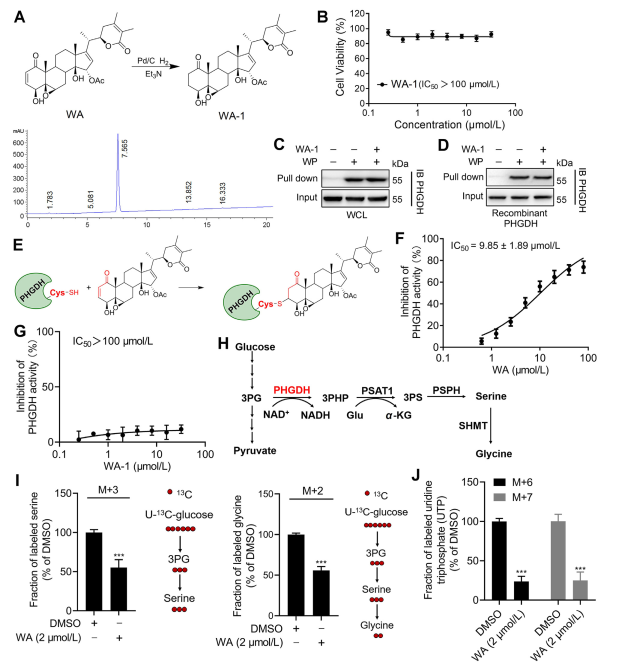
Figure 3: WA Covalently Binds to PHGDH and Inhibits PHGDH Activity.
4. The Cys295 Residue of PHGDH is Covalently Bound to WA.
Theoretically, the α and β-unsaturated ketone moieties of WA may form a covalent bond with cysteine residues of the protein. Indeed, the authors observed covalent modification of the Cys295 residues in PHGDH by WA. Subsequently, they provided further evidence of WA's covalent binding to the Cys295 residues of PHGDH and demonstrated that mutations at Cys295 significantly reduced WA's inhibitory activity on PHGDH. Additionally, Biolayer Interferometry (BLI) experiments revealed no interaction between WA and recombinant Cys295A PHGDH protein, confirming the selective covalent binding of WA to PHGDH.
Moreover, molecular dynamics simulations indicated that WA functions as an allosteric regulator of PHGDH, with the Cys295 residue potentially serving as a novel allosteric site for PHGDH.
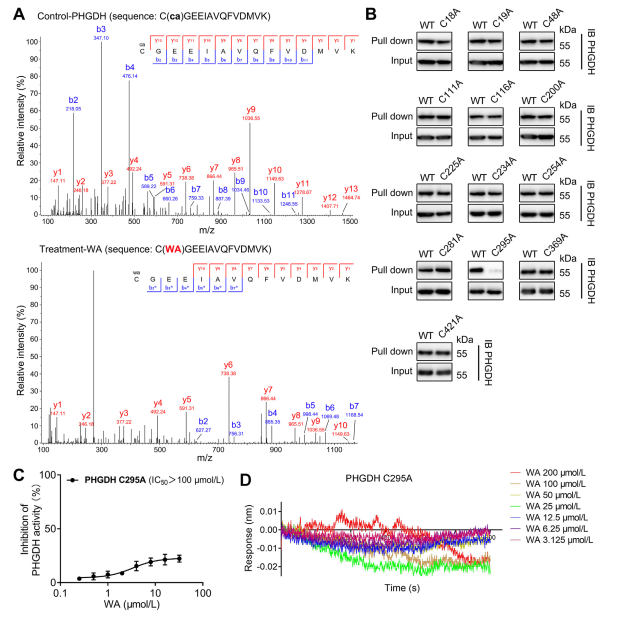
Figure 4. WA Selectively covalently binds to the Cys295 residues of PHGDH.
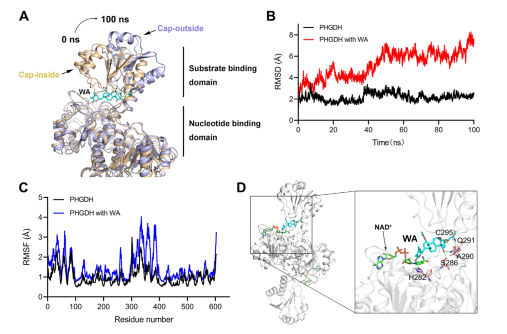
Figure 5: Cys295 Residue as the Allosteric Regulatory Site of PHGDH
5. The Effect of WA on Redox Balance in HCT-116 Cells.
The serine synthesis pathway (SSP) plays a pivotal role in maintaining cellular redox balance by providing precursors for glutathione (GSH) synthesis and NADPH production, essential for redox reactions. The authors investigated the impact of WA on redox balance in HCT-116 cells and observed that WA led to increased levels of reactive oxygen species (ROS), consequently reducing the GSH/GSSG and NADPH/NADP+ ratios, indicative of heightened oxidative stress.
Western blot analysis revealed elevated expression of γ H2AX, Cleaved caspase3, and cleaved PARP, along with decreased CHK2 and cyclin D1 expression, suggesting induction of apoptosis by WA. Moreover, loss of PHGDH resulted in increased ROS production, inhibited proliferation of HCT-116 cells, and reduced cytotoxicity of WA in cells with low PHGDH expression. These findings underscore the role of PHGDH in ROS production and WA-mediated cytotoxicity in HCT-116 cells.
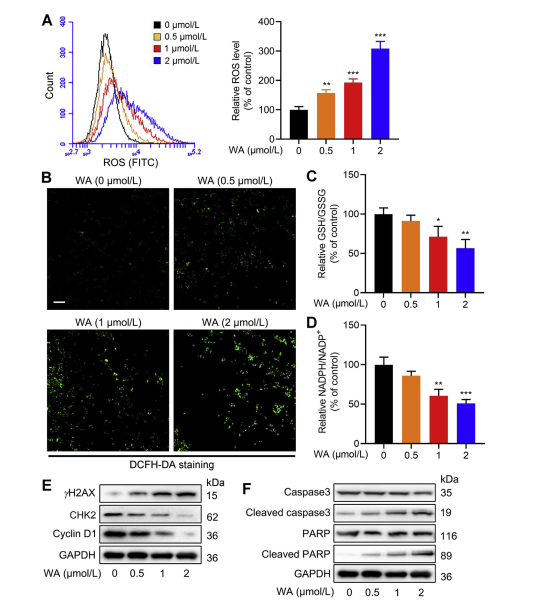
Figure 6: WA Increases Intracellular ROS Levels in HCT-116 Cells
6. Cell Proliferation Effect in Xenograft Models.
To assess the impact of WA on the proliferation of HCT-116 cells in vivo, the authors established a xenograft model using HCT-116 cells. The results revealed that WA exhibited no significant effects on body weight or organ morphology, indicating low toxicity.
To further investigate the selectivity of WA for PHGDH inhibition, the authors generated a xenograft model using PHGDH knockout (KO) HCT116 cells. In this model, the absence of PHGDH markedly inhibited HCT-116 cell proliferation (refer to Figure 7, A-C). Additionally, the expression of Ki67, a marker of cell proliferation, was significantly reduced in the PHGDH KO HCT116 cell xenograft model (refer to Figure 7, D).
Notably, in the xenograft model using PHGDH KO HCT-116 cells, WA demonstrated no significant inhibitory effect on cell proliferation (refer to Figure 7, E-G), further confirming that the inhibitory effect of WA on HCT-116 cells depended on PHGDH.
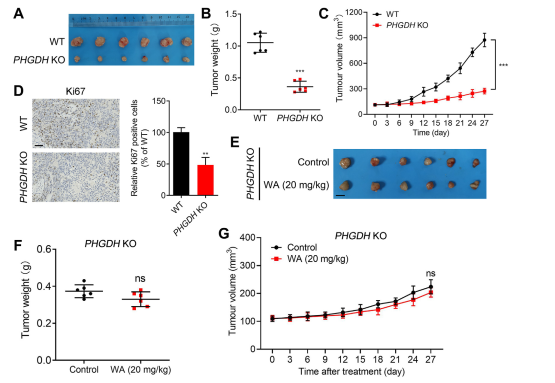
Figure 7: Effect of WA on the proliferation of PHGDH KO HCT-116 cells in vivo
The results of this study not only provide key clues for the future anticancer drug development for PHGDH, but also bring new hope and potential for the new drug development in the field of cancer therapy.
Reference: https://doi.org/10.1016/j.apsb.2021.06.008