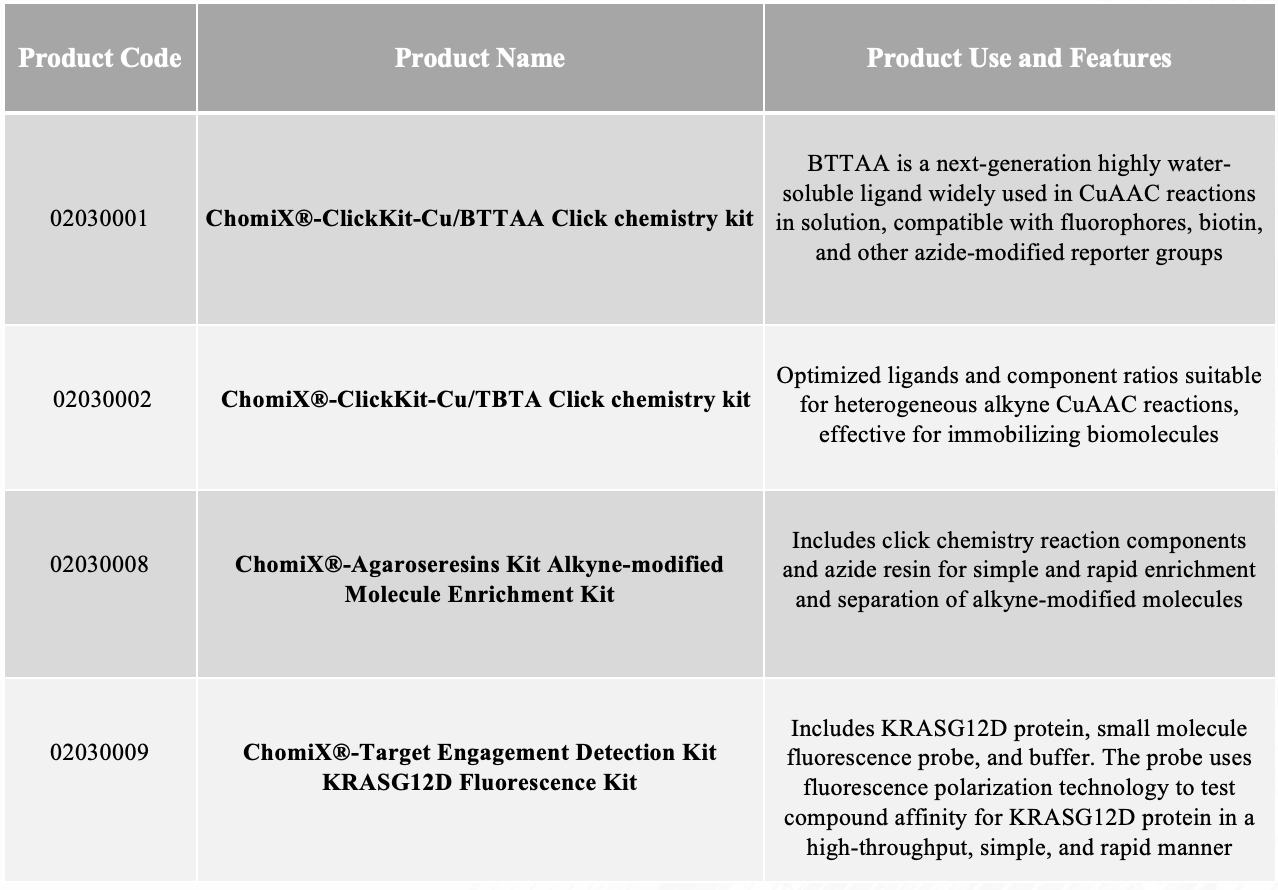
Products
Bioorthogonal Coupling Reaction Kit
Introduction
There are various types of bioorthogonal coupling reactions, among which the most widely used are the Staudinger ligation, copper-catalyzed azide-alkyne cycloaddition (CuAAC), strain-promoted [3+2] cycloaddition (SPAAC), and tetrazine-involved Diels-Alder reaction (IEDDA).
In the CuAAC reaction, a 1,4-disubstituted triazole linkage is formed, which is similar in size and geometry to an amide bond, exhibiting high thermal and chemical stability. This reaction can proceed rapidly in aqueous conditions, typically without producing byproducts, and is compatible with most functional groups. By controlling different ligands, reducing agents, and ratios, the alkyne-azide coupling reaction can be efficiently catalyzed in both homogeneous and heterogeneous reaction systems, and applied in protein fluorescent labeling, solid-phase carrier separation, among other areas. Copper-free reactions, including SPAAC and IEDDA, are particularly suitable for live cell systems, causing minimal damage to cells, hence finding application in live cell imaging, cell surface glycan labeling, and more.
Furthermore, we has also developed a variety of magnetic bead products for the enrichment and separation of biomolecules modified with chemical probes.